How PROMACTA May Work for Your Child
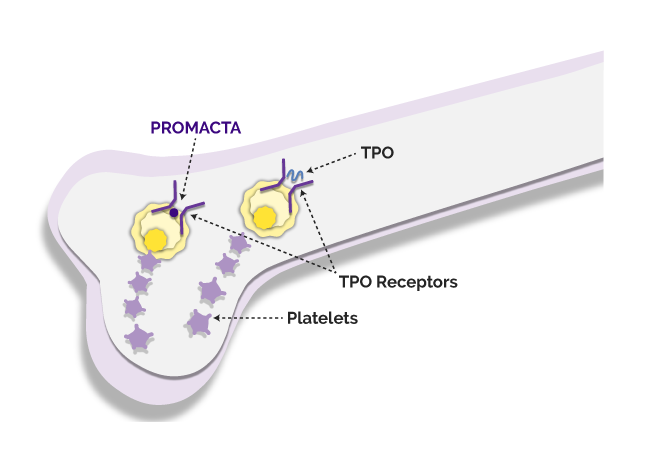
PROMACTA is a platelet booster, also known as a TPO-RA (thrombopoietin receptor agonist). This means that it boosts the number of platelets your child’s body makes.
PROMACTA is the only once-daily oral platelet booster that is available as a tablet and an oral suspension. It is also the only oral platelet booster approved to treat persistent or chronic immune thrombocytopenia (ITP) in children 1 year and older.
How PROMACTA treats persistent or chronic ITP
A hormone called thrombopoietin (TPO) naturally creates platelets in the body and, unlike some other drugs used to treat persistent or chronic ITP, PROMACTA acts the same as TPO—working alongside your body to create more platelets.
PROMACTA is the only once-daily oral platelet booster that comes in both a tablet and an oral suspension, so even if your child can’t swallow a pill, they can still receive a once-daily oral therapy. Some other treatments for persistent or chronic ITP require doctor visits for an injection, while PROMACTA can be taken at home, at school, or on the go!
What makes PROMACTA different?
For pediatric patients, it's the only once-daily oral TPO-RA treatment for persistent or chronic ITP, and it has the following features:
PROMACTA was studied in more pediatric patients than any other drug of its kind
PROMACTA has been proven to help pediatric patients with persistent or chronic immune thrombocytopenia (ITP) reach target platelet levels and maintain them for a long time.
Primary End Point
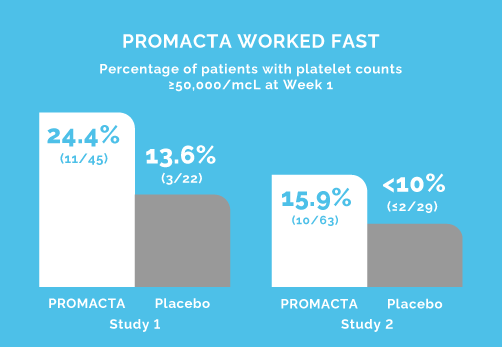
PETIT (Study 1): The primary end point was the proportion of patients achieving platelet counts ≥50,000/mcL at least once between Days 8 and 43 (Weeks 1 to 6) of the randomized period.
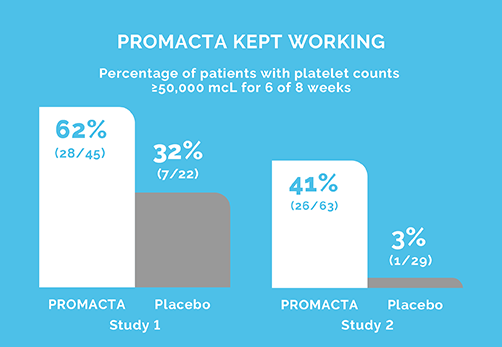
PETIT 2 (Study 2): The primary end point was the proportion of patients achieving platelet counts ≥50,000/mcL at least 6 of the 8 weeks between Weeks 5 to 12 of the randomized period.
Study Design
PETIT (Study 1): Open-label, double blind, randomized, placebo-controlled study to investigate the efficacy, safety, tolerability, and pharmacokinetics of eltrombopag in previously treated pediatric patients (n=67) with persistent or chronic ITP.
PETIT 2 (Study 2): Open-label, double-blind, randomized, placebo-controlled study to investigate the efficacy, safety, and tolerability of eltrombopag in previously treated pediatric patients (n=92) with chronic ITP.
PROMACTA helped in other ways
13% of patients (6 of 45) on PROMACTA needed to take rescue medication to raise their platelet levels compared with 50% of patients (11 of 22) on placebo
- In a second study, 19% (12 of 63) on PROMACTA needed rescue medication versus 24% (7 of 29) on placebo
53% of patients (8 of 15) taking other ITP medications were able to reduce or stop using them after starting PROMACTA
- In a second study, 46% of patients (6 of 13) taking PROMACTA reduced or stopped other ITP medications